DCGI approves use of Russian vaccine 'Sputnik V'; India gets 3rd vaccine
India gets a third COVID-19 vaccine as the Drug Controller General of India (DCGI) gave its approval to Russian vaccine Sputnik V for emergency use in India against coronavirus, Russian Direct Investment Fund said on Monday.

It is pertinent to mention that India is, currently, having two vaccines — Covaxin and COVISHIELD. It may be recalled that Russia claimed 'Sputnik V' to the world's first COVID-19 vaccine against the contagious disease.
Also Read | With 1.68 lakh new coronavirus cases, India records another new daily high
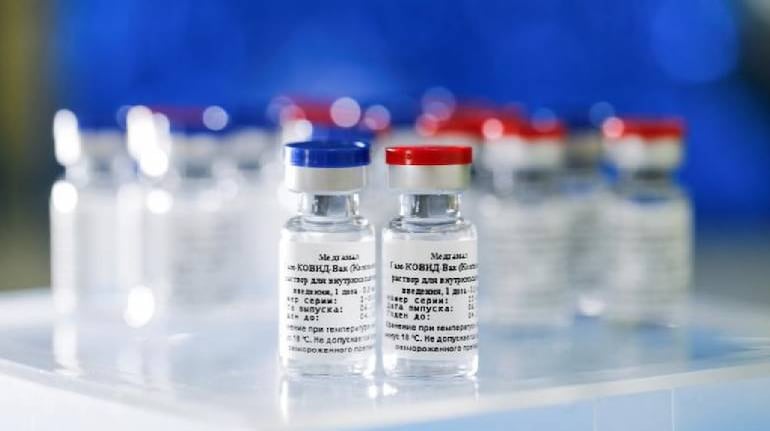
Last week, Dr Reddy's Laboratories, the Hyderabad-based pharmaceutical company had sought the government's approval for the use of its vaccine in India.

As per the report, it has an efficacy of 91.6 percent based on the interim analysis of phase III clinical trials.
Also Read | Covid-19 vaccine is need of country: Rahul Gandhi
These included data from 19,866 volunteers in Russia. The approval comes at a time when India is facing a surge in Covid-19 cases across the country with Maharashtra, Punjab, Karnataka, Delhi, Uttar Pradesh, Chhattisgarh, Karnataka, and Kerala witnessing a major surge in daily caseload.

India launched its vaccination drive on January 16 with Covaxin and Covishield after they granted emergency use approval on January 1 and January 3, respectively.
Click here to follow PTC News on Twitter
