No Covid vaccine for children yet as regulatory approval awaited: Bharat Biotech
Jasleen Kaur
October 12th 2021 08:45 PM --
Updated:
October 12th 2021 08:57 PM

The Subject Expert Committee (SEC) has given a recommendation to the Drugs Controller General of India (DCGI) for the use of Bharat Biotech's Covid-19 vaccine, Covaxin, for the beneficiaries aged between 2-18 years, the official sources said on Tuesday.

Also read | After banning coal-based power plants in Delhi, Arvind Kejriwal cries 'coal shortage'
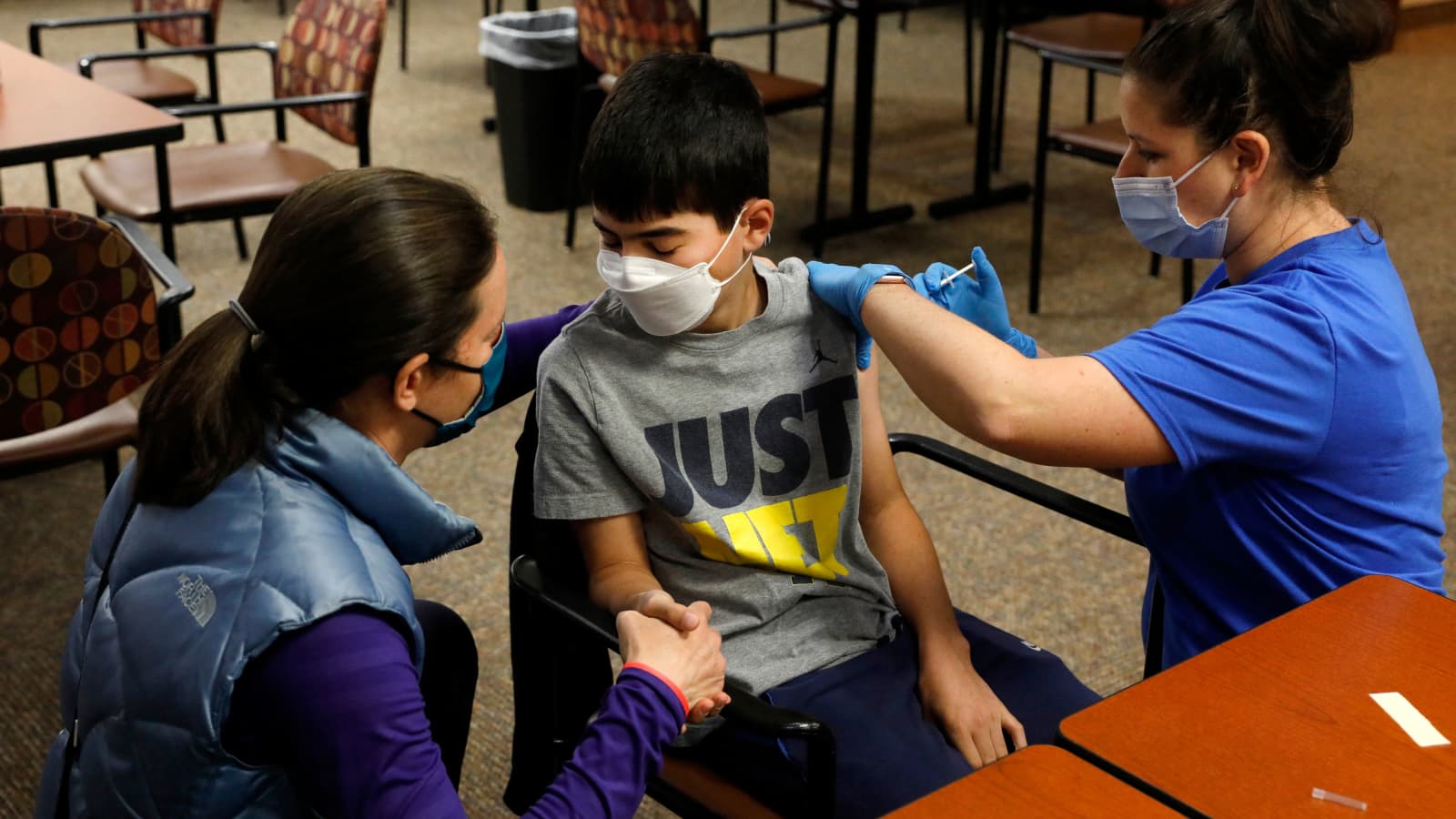 After evaluation of the data, the final approval will be given by the Drug Controller General of India (DCGI). Bharat Biotech in an official statement has said they are waiting for further approvals from drug regulators.
"This represents one of the first approvals worldwide for Covid-19 vaccines for children in the 2-18 age group. Bharat Biotech sincerely thanks the DCGI, Subject Experts Committee, and Central Drugs Standard Control Organisation (CDSCO) for their expedited review process. We now await further regulatory approvals from the CDSCO prior to product launch and market availability of Covaxin for children," stated the statement issued by Bharat Biotech.
After evaluation of the data, the final approval will be given by the Drug Controller General of India (DCGI). Bharat Biotech in an official statement has said they are waiting for further approvals from drug regulators.
"This represents one of the first approvals worldwide for Covid-19 vaccines for children in the 2-18 age group. Bharat Biotech sincerely thanks the DCGI, Subject Experts Committee, and Central Drugs Standard Control Organisation (CDSCO) for their expedited review process. We now await further regulatory approvals from the CDSCO prior to product launch and market availability of Covaxin for children," stated the statement issued by Bharat Biotech.
 Also read | Jammu and Kashmir: Five LeT terrorists killed by security forces in Shopian
This is one of the first worldwide approval for Covid-19 vaccines for the age group of beneficiaries belonging to 2-18 years.
Also read | Jammu and Kashmir: Five LeT terrorists killed by security forces in Shopian
This is one of the first worldwide approval for Covid-19 vaccines for the age group of beneficiaries belonging to 2-18 years.
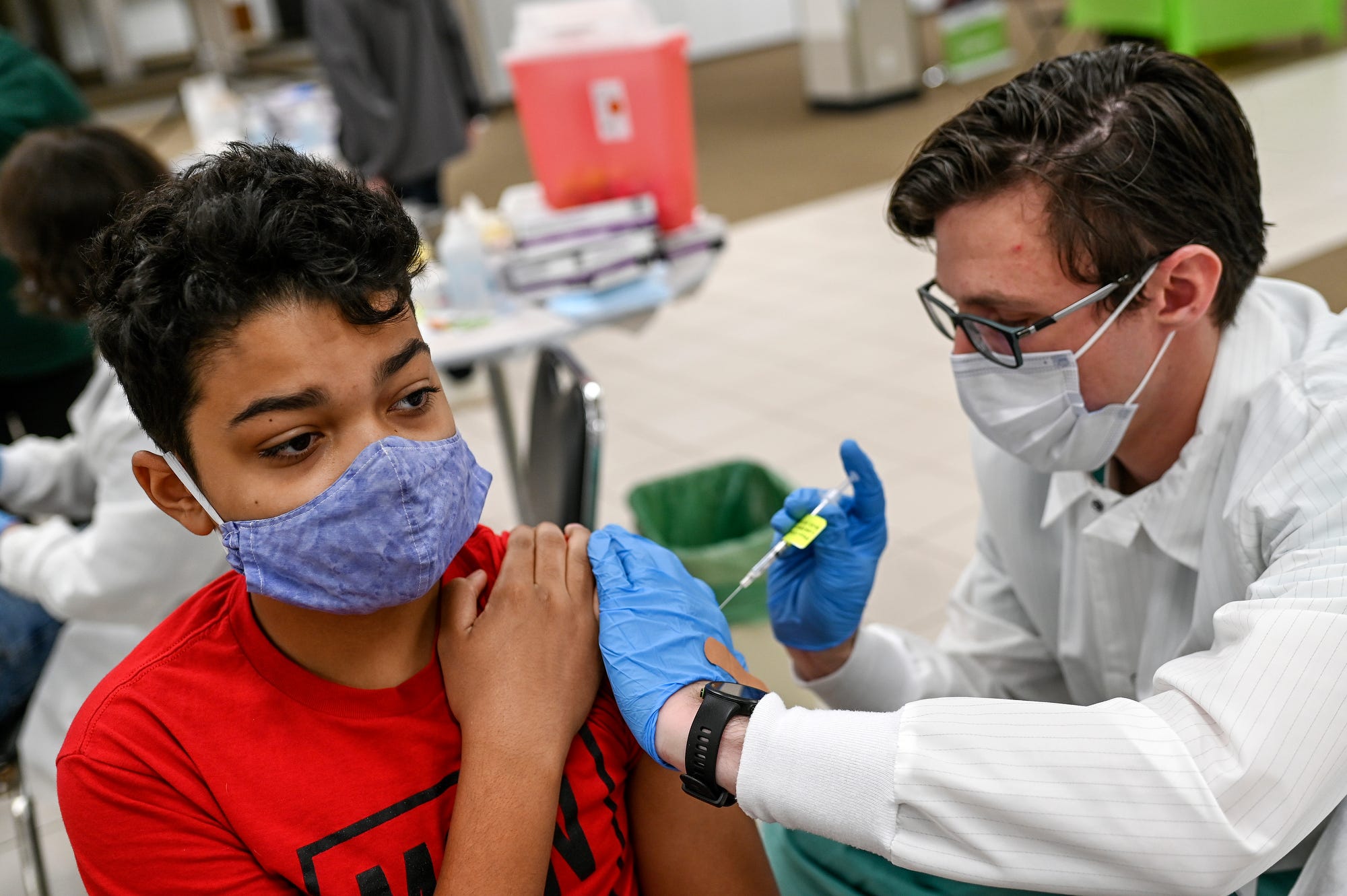 Bharat Biotech has submitted data from clinical trials in the 2-18 years age group for Covaxin (BBV152) to CDSCO. The data has been thoroughly reviewed by the CDSCO and Subject Experts Committee (SEC) and have provided their positive recommendations, the vaccine maker said. The Subject Expert Committee on Covid-19 examined the data on Monday.
Bharat Biotech has submitted data from clinical trials in the 2-18 years age group for Covaxin (BBV152) to CDSCO. The data has been thoroughly reviewed by the CDSCO and Subject Experts Committee (SEC) and have provided their positive recommendations, the vaccine maker said. The Subject Expert Committee on Covid-19 examined the data on Monday.
 -PTC News
-PTC News
 After evaluation of the data, the final approval will be given by the Drug Controller General of India (DCGI). Bharat Biotech in an official statement has said they are waiting for further approvals from drug regulators.
"This represents one of the first approvals worldwide for Covid-19 vaccines for children in the 2-18 age group. Bharat Biotech sincerely thanks the DCGI, Subject Experts Committee, and Central Drugs Standard Control Organisation (CDSCO) for their expedited review process. We now await further regulatory approvals from the CDSCO prior to product launch and market availability of Covaxin for children," stated the statement issued by Bharat Biotech.
After evaluation of the data, the final approval will be given by the Drug Controller General of India (DCGI). Bharat Biotech in an official statement has said they are waiting for further approvals from drug regulators.
"This represents one of the first approvals worldwide for Covid-19 vaccines for children in the 2-18 age group. Bharat Biotech sincerely thanks the DCGI, Subject Experts Committee, and Central Drugs Standard Control Organisation (CDSCO) for their expedited review process. We now await further regulatory approvals from the CDSCO prior to product launch and market availability of Covaxin for children," stated the statement issued by Bharat Biotech.
 Also read | Jammu and Kashmir: Five LeT terrorists killed by security forces in Shopian
This is one of the first worldwide approval for Covid-19 vaccines for the age group of beneficiaries belonging to 2-18 years.
Also read | Jammu and Kashmir: Five LeT terrorists killed by security forces in Shopian
This is one of the first worldwide approval for Covid-19 vaccines for the age group of beneficiaries belonging to 2-18 years.
 -PTC News
-PTC News


