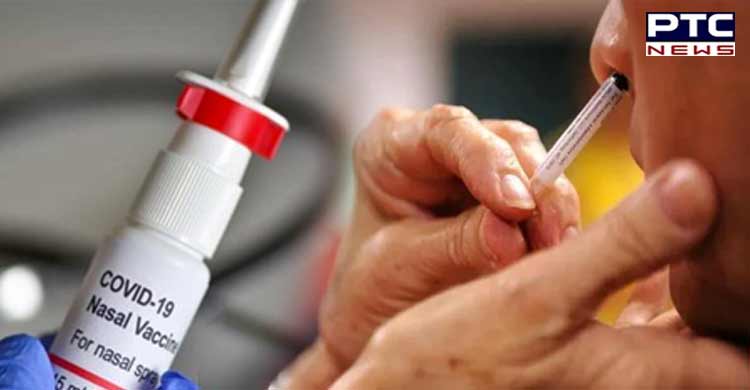
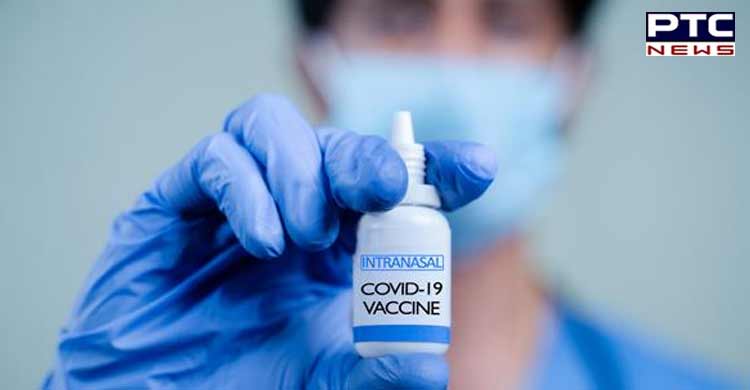
DCGI nod to Bharat Biotech to conduct clinical trials of intranasal Covid-19 vaccine BBV154
New Delhi, January 28: The Drugs Controller General of India (DCGI) on Friday granted permission to Bharat Biotech to conduct clinical trials of intranasal vaccine BBV154 against Covid-19.
 The Subject Expert Committee (SEC) of the Central Drugs Standard Control Organisation (CDSCO) had also recommended the internasal Covid-19 vaccine clinical trials during the last meeting.
The NOC letter issued by the DCGI read, "Central Licensing Authority hereby permits Bharat Biotech International limited to conduct clinical trials of the new drug or investigational new drug."
The Subject Expert Committee (SEC) of the Central Drugs Standard Control Organisation (CDSCO) had also recommended the internasal Covid-19 vaccine clinical trials during the last meeting.
The NOC letter issued by the DCGI read, "Central Licensing Authority hereby permits Bharat Biotech International limited to conduct clinical trials of the new drug or investigational new drug."
 Also read | Army aircraft crashes in Bihar's Gaya, pilots safe
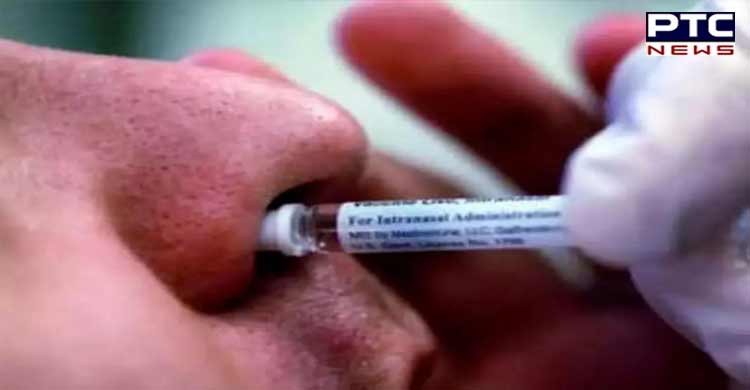
The dosage form of the Covid-19 nasal vaccine is liquid for the intranasal route of administration and every single dose contains 0.5ml. The trials of the Intranasal vaccine will take place at nine different sites of the country.
The trials will be conducted at Atman Hospital, Ahmedabad, Gujarat; AIIMS, Delhi and Patna; Oyster and Pearls Hospitals, Pune; Pt.BD Sharma Postgraduate Institute of Medical Sciences at Rohtak, Haryana; Acharya Vinobha Bhave Rural Hospital, Wardha; Jeevan Rekha Hospital, Belagavi; Rana hospital, Gorakhpur; and Prakhar Hospital, Uttar Pradesh.
"The Phase -3 clinical trial should be conducted as per protocol multi-center study to compare immunogenicity and safety of BBV154 with COVAXIN." read the letter.
Meanwhile, Union Health Minister Mansukh Mandaviya in a Covid-19 review meeting with Southern states and UTs Health Minister on Friday stressed upon e-Sanjeevani, teleconsultation, monitoring, home isolation and increasing RT-PCR tests in states which are reporting lower percentage testing.
Also read | Army aircraft crashes in Bihar's Gaya, pilots safe
The dosage form of the Covid-19 nasal vaccine is liquid for the intranasal route of administration and every single dose contains 0.5ml. The trials of the Intranasal vaccine will take place at nine different sites of the country.
The trials will be conducted at Atman Hospital, Ahmedabad, Gujarat; AIIMS, Delhi and Patna; Oyster and Pearls Hospitals, Pune; Pt.BD Sharma Postgraduate Institute of Medical Sciences at Rohtak, Haryana; Acharya Vinobha Bhave Rural Hospital, Wardha; Jeevan Rekha Hospital, Belagavi; Rana hospital, Gorakhpur; and Prakhar Hospital, Uttar Pradesh.
"The Phase -3 clinical trial should be conducted as per protocol multi-center study to compare immunogenicity and safety of BBV154 with COVAXIN." read the letter.
Meanwhile, Union Health Minister Mansukh Mandaviya in a Covid-19 review meeting with Southern states and UTs Health Minister on Friday stressed upon e-Sanjeevani, teleconsultation, monitoring, home isolation and increasing RT-PCR tests in states which are reporting lower percentage testing.
 The Union Health Minister conducted a high-level meeting on Friday through video conferencing in the context of the Omicron variant of SARS-CoV-2 with Health Ministers of southern states/UT's Andhra Pradesh, Karnataka, Kerala, Telangana, Lakshadweep, Tamil Nadu, Puducherry and Andaman and Nicobar Island. The meeting was also attended by senior health officials.
Also read | Centre extends Covid-19 restrictions till February 28
Earlier, he conducted a high-level meeting with nine Northern states and UTs and advised them to send Covid testing and vaccination data timely.
The Union Health Minister conducted a high-level meeting on Friday through video conferencing in the context of the Omicron variant of SARS-CoV-2 with Health Ministers of southern states/UT's Andhra Pradesh, Karnataka, Kerala, Telangana, Lakshadweep, Tamil Nadu, Puducherry and Andaman and Nicobar Island. The meeting was also attended by senior health officials.
Also read | Centre extends Covid-19 restrictions till February 28
Earlier, he conducted a high-level meeting with nine Northern states and UTs and advised them to send Covid testing and vaccination data timely.
 -PTC News
-PTC News
