
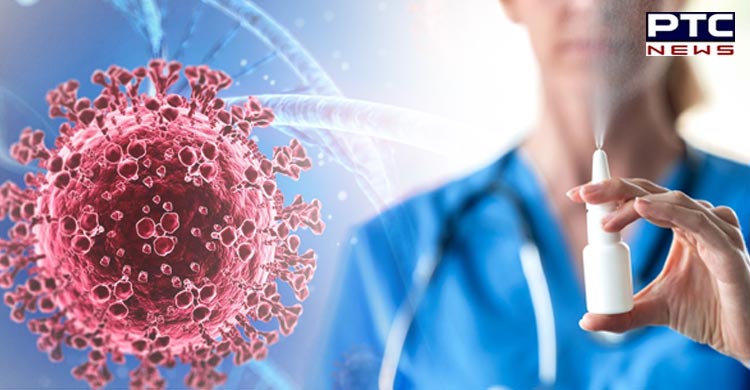
Glenmark launches first nasal spray for adult Covid-19 patients in India
New Delhi, February 9: Mumbai based global pharma company Glenmark launched Nitric Oxide Nasal Spray (FabiSpray) in India, in partnership with SaNOtize, for treatment of adult Covid-19 patients.
 As part of the accelerated approval procedure, Glenmark acquired manufacturing and marketing authorisation for Nitric Oxide Nasal Spray from India's drug regulator, Drugs Controller General of India (DCGI).
As per the official statement, "Phase 3 trial in India met the key endpoints and demonstrated reduction of viral load of 94 per cent in 24 hours and 99 per cent in 48 hours. Nitric Oxide Nasal Spray (NONS) was safe and well-tolerated in Covid-19 patients. Glenmark to market NONS under the brand name FabiSpray."
As part of the accelerated approval procedure, Glenmark acquired manufacturing and marketing authorisation for Nitric Oxide Nasal Spray from India's drug regulator, Drugs Controller General of India (DCGI).
As per the official statement, "Phase 3 trial in India met the key endpoints and demonstrated reduction of viral load of 94 per cent in 24 hours and 99 per cent in 48 hours. Nitric Oxide Nasal Spray (NONS) was safe and well-tolerated in Covid-19 patients. Glenmark to market NONS under the brand name FabiSpray."
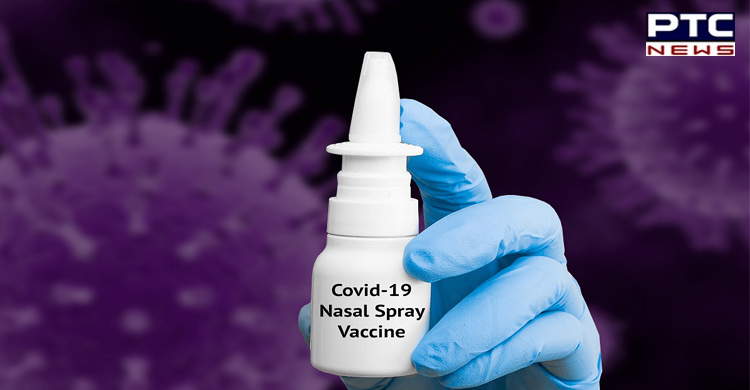 The firm claims that when the Nitric Oxide Nasal is sprayed over nasal mucosa it serves as a physical and chemical barrier against the Covid-19 virus.
Also Read | Covid-19: Over 11.81 cr unutilised vaccine doses still available with states, UTs
"FabiSpray is designed to kill the Covid-19 virus in the upper airways. It has proven anti-microbial properties with a direct virucidal effect on SARS-CoV-2. NONS when sprayed over nasal mucosa acts as a physical and chemical barrier against the virus, preventing it from incubating and spreading to the lungs," the statement further read.
Glenmark Pharmaceuticals Ltd's Chief Commercial Officer Robert Crockart described the spray as an effective and safe antiviral treatment for Covid-19, adding, "we are confident it will offer patients a much needed and timely therapy option."
The firm claims that when the Nitric Oxide Nasal is sprayed over nasal mucosa it serves as a physical and chemical barrier against the Covid-19 virus.
Also Read | Covid-19: Over 11.81 cr unutilised vaccine doses still available with states, UTs
"FabiSpray is designed to kill the Covid-19 virus in the upper airways. It has proven anti-microbial properties with a direct virucidal effect on SARS-CoV-2. NONS when sprayed over nasal mucosa acts as a physical and chemical barrier against the virus, preventing it from incubating and spreading to the lungs," the statement further read.
Glenmark Pharmaceuticals Ltd's Chief Commercial Officer Robert Crockart described the spray as an effective and safe antiviral treatment for Covid-19, adding, "we are confident it will offer patients a much needed and timely therapy option."
 He further added that "As a leading pharmaceutical player, it is important that we are an integral part of India's fight against the Covid-19 pandemic. We are happy to receive regulatory approval for Nitric Oxide Nasal Spray (FabiSpray) and launch it in partnership with SaNOtize."
Dr Monika Tandon, Senior VP and Head of Clinical Development at Glenmark Pharmaceuticals Ltd., commented on the issue of clinical trial results, saying, "The results from this Phase 3, double-blind, placebo-controlled trial are encouraging. Demonstration of reduction in the viral load has a significant positive impact from a patient and community perspective. In the current scenario, with new emerging variants exhibiting high transmissibility, NONS provides a useful option in India's fight against Coronavirus."
"According to research conducted at Utah State University in the United States, NONS is proven to kill 99.9% of SARS-Cov-2 virus strains in about 2 minutes, including Alpha, Beta, Gamma, Delta, and Epsilon," she added.
He further added that "As a leading pharmaceutical player, it is important that we are an integral part of India's fight against the Covid-19 pandemic. We are happy to receive regulatory approval for Nitric Oxide Nasal Spray (FabiSpray) and launch it in partnership with SaNOtize."
Dr Monika Tandon, Senior VP and Head of Clinical Development at Glenmark Pharmaceuticals Ltd., commented on the issue of clinical trial results, saying, "The results from this Phase 3, double-blind, placebo-controlled trial are encouraging. Demonstration of reduction in the viral load has a significant positive impact from a patient and community perspective. In the current scenario, with new emerging variants exhibiting high transmissibility, NONS provides a useful option in India's fight against Coronavirus."
"According to research conducted at Utah State University in the United States, NONS is proven to kill 99.9% of SARS-Cov-2 virus strains in about 2 minutes, including Alpha, Beta, Gamma, Delta, and Epsilon," she added.
 Dr Srikanth Krishnamurthy, one of the study's Principal Researchers, stated, "I have had a chance to view the results of the study. Nitric Oxide Nasal Spray lowers the viral load and hastens RT-PCR negativity when used early in Covid infection leading to recovery. Most importantly, viral load reduction with NONS has the potential to reduce the chain of transmission. Last but not the least, NONS being topical is safe and makes this therapeutic option very attractive".
Also Read | T20 World Cup 2022: India-Pak match tickets sold out within minutes
-PTC News
Dr Srikanth Krishnamurthy, one of the study's Principal Researchers, stated, "I have had a chance to view the results of the study. Nitric Oxide Nasal Spray lowers the viral load and hastens RT-PCR negativity when used early in Covid infection leading to recovery. Most importantly, viral load reduction with NONS has the potential to reduce the chain of transmission. Last but not the least, NONS being topical is safe and makes this therapeutic option very attractive".
Also Read | T20 World Cup 2022: India-Pak match tickets sold out within minutes
-PTC News
