

Pfizer seeks emergency use authorisation for COVID-19 vaccine in India
Pfizer and BioTech's mRNA vaccine BNT162b2: Pfizer India has become the first pharmaceutical firm to seek an emergency use authorisation for its COVID-19 vaccine in India from the Drugs Controller General of India (DCGI).
 The development comes after its parent company got the clearance in the UK and Bahrain.
Also Read | ‘Kisan ki mange puri karo’: Diljit Dosanjh spotted at farmers protest at Singhu border
The development comes after its parent company got the clearance in the UK and Bahrain.
Also Read | ‘Kisan ki mange puri karo’: Diljit Dosanjh spotted at farmers protest at Singhu border
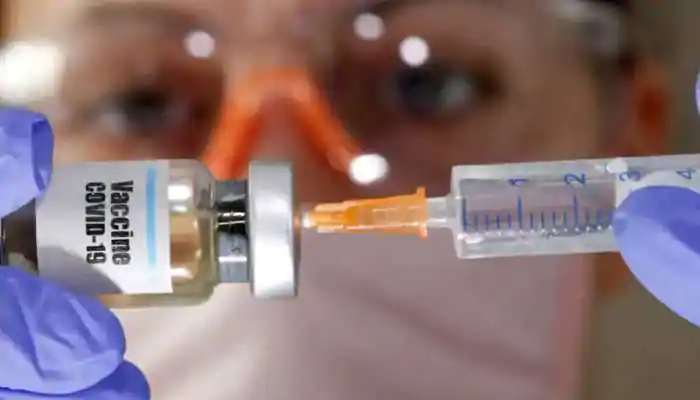 In an application submitted to the drug regulator, it sought permission to import the Pfizer-BioTech's mRNA vaccine BNT162b2 for the sale and distribution in the country.
Also, the firm sought a waiver of clinical trials on the Indian population in accordance with the special provisions under the New Drugs and Clinical Trials Rules, 2019, the official sources said.
In an application submitted to the drug regulator, it sought permission to import the Pfizer-BioTech's mRNA vaccine BNT162b2 for the sale and distribution in the country.
Also, the firm sought a waiver of clinical trials on the Indian population in accordance with the special provisions under the New Drugs and Clinical Trials Rules, 2019, the official sources said.
 As per the sources, Pfizer India submitted an application on December 4 to the DCGI seeking emergency use authorization (EUA).
As per the sources, Pfizer India submitted an application on December 4 to the DCGI seeking emergency use authorization (EUA).
 The firm also submitted the EUA application in Form CT-18 for permission to import and market Pfizer-BioNTech's COVID-19 mRNA vaccine BNT162b2 in India.
Also Read | Gold price crashes minutes after Pfizer says COVID-19 vaccine 90 percent effective
Earlier on Wednesday, the UK became the first country to approve the Pfizer/BioNTech vaccine against COVID-19 as the UK regulator Medicines and Healthcare products Regulatory Agency (MHRA) granted a temporary authorisation for its emergency use.
The firm also submitted the EUA application in Form CT-18 for permission to import and market Pfizer-BioNTech's COVID-19 mRNA vaccine BNT162b2 in India.
Also Read | Gold price crashes minutes after Pfizer says COVID-19 vaccine 90 percent effective
Earlier on Wednesday, the UK became the first country to approve the Pfizer/BioNTech vaccine against COVID-19 as the UK regulator Medicines and Healthcare products Regulatory Agency (MHRA) granted a temporary authorisation for its emergency use.
 The jab claims to offer up to 95 percent efficiency against COVID-19 and is touted as safe for roll-out.
The jab claims to offer up to 95 percent efficiency against COVID-19 and is touted as safe for roll-out.
 Similarly, Bahrain, on Friday, announced that it has granted a EUA for the two-dose vax by Pfizer and BioNTech.
-PTC News
Similarly, Bahrain, on Friday, announced that it has granted a EUA for the two-dose vax by Pfizer and BioNTech.
-PTC News
