Washington, April 16: The Food and Drug Administration (FDA) authorised the first test, InspectIR Covid-19 Breathalyzer, to detect Covid-19 through the breath for emergency use.


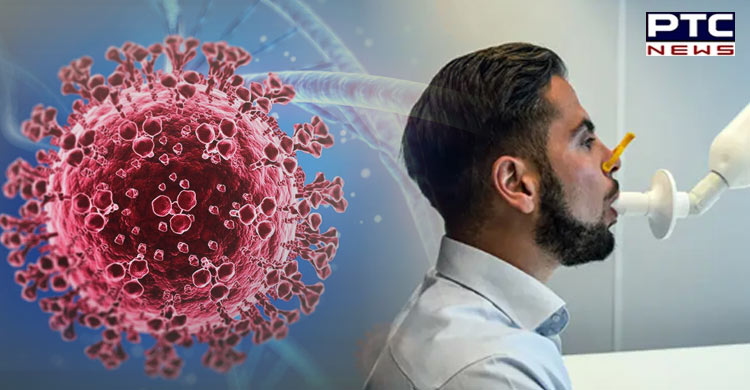
FDA authorises first-ever breathing test for detecting Covid-19 infection
Washington, April 16: The Food and Drug Administration (FDA) authorised the first test, InspectIR Covid-19 Breathalyzer, to detect Covid-19 through the breath for emergency use.

 The InspectIR Covid-19 Breathalyzer which is about the size of a carry-on piece of luggage is able to identify five volatile organic compounds tied to the coronavirus in a person's breath using a technique known as gas chromatography gas mass-spectrometry, delivering results within three minutes, according to the FDA, the statement reads.
Also read | Punjab: AAP govt announces 300 units of free power for homes from July 1
The agency said in a study of 2,409 persons, which included both people with and without symptoms, the test had a 99.3 per cent specificity rate, which measures the per cent of correctly identified negative test samples.
The InspectIR Covid-19 Breathalyzer which is about the size of a carry-on piece of luggage is able to identify five volatile organic compounds tied to the coronavirus in a person's breath using a technique known as gas chromatography gas mass-spectrometry, delivering results within three minutes, according to the FDA, the statement reads.
Also read | Punjab: AAP govt announces 300 units of free power for homes from July 1
The agency said in a study of 2,409 persons, which included both people with and without symptoms, the test had a 99.3 per cent specificity rate, which measures the per cent of correctly identified negative test samples.
 The FDA also noted that the InspectIR COVID-19 Breathalyzer had a 91.2 per cent sensitivity rate, which measures the per cent of correctly identified positive test samples.
However, the health agency said that a molecular test should be used to confirm positive test results returned by the COVID-19 breath test.
Jeff Shuren, director of the FDA's Center for Devices and Radiological Health, in a statement, said, "Today's authorization is yet another example of the rapid innovation occurring with diagnostic tests for COVID-19," as per The Hill.
Nearly 100 of the InspectIR COVID-19 Breathalyzers, each of which the FDA said can be used to test roughly 160 samples per day, are anticipated to be made each week.
Also read | CBSE to restore single board exam pattern for Classes X, XII from next session
The FDA also noted that the InspectIR COVID-19 Breathalyzer had a 91.2 per cent sensitivity rate, which measures the per cent of correctly identified positive test samples.
However, the health agency said that a molecular test should be used to confirm positive test results returned by the COVID-19 breath test.
Jeff Shuren, director of the FDA's Center for Devices and Radiological Health, in a statement, said, "Today's authorization is yet another example of the rapid innovation occurring with diagnostic tests for COVID-19," as per The Hill.
Nearly 100 of the InspectIR COVID-19 Breathalyzers, each of which the FDA said can be used to test roughly 160 samples per day, are anticipated to be made each week.
Also read | CBSE to restore single board exam pattern for Classes X, XII from next session
 -PTC News
-PTC News
