
Novavax vaccine demonstrates 90% efficacy, 100% protection against moderate, severe disease
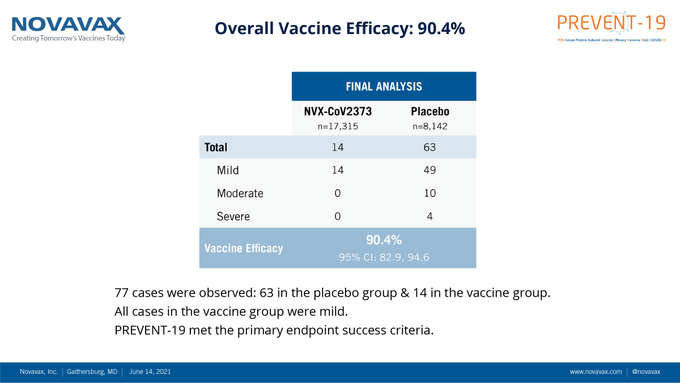
The US company Novavax said that its COVID-19 vaccine, in the Phase 3 trial, demonstrated an overall efficacy of 90.4 percent against mild, moderate, and severe disease caused by the COVID-19 strain.
 "Novavax COVID-19 Vaccine demonstrates 90% overall efficacy and 100% protection against moderate and severe disease in PREVENT-19 Phase 3 Trial," the company said in a tweet.
Also Read | Coronavirus: India reports 70,421 new cases in 24 hours; lowest after 74 days
"Novavax COVID-19 Vaccine demonstrates 90% overall efficacy and 100% protection against moderate and severe disease in PREVENT-19 Phase 3 Trial," the company said in a tweet.
Also Read | Coronavirus: India reports 70,421 new cases in 24 hours; lowest after 74 days

 Stanley C. Erck, President and Chief Executive Officer, Novavax, said that "Novavax is one step closer to addressing the critical and persistent global public health need for additional COVID-19 vaccines."
Biotech firm is planning to file for authorisation with the Food and Drug Administration in the third quarter of 2021.
Click here to follow PTC News on Twitter
-PTC News
Stanley C. Erck, President and Chief Executive Officer, Novavax, said that "Novavax is one step closer to addressing the critical and persistent global public health need for additional COVID-19 vaccines."
Biotech firm is planning to file for authorisation with the Food and Drug Administration in the third quarter of 2021.
Click here to follow PTC News on Twitter
-PTC News 